Background:Immune thrombocytopenia (ITP) is an acquired autoimmune disorder, characterized by increased platelet destruction and impaired platelet production. Therefore, affected patients present with bleeding complications of various severity. However, another frequent complication of ITP is fatigue, which is often underestimated. In Europe the oral thrombopoietin receptor agonist Eltrombopag (EPAG) is licensed for the treatment of patients with persistent and chronic ITP who are refractory to previous treatments. EPAG has previously been shown to elevate platelet count and reduce bleeding complications in ITP patients, but the therapeutic effect on fatigue is unclear. Here we present data from the scheduled 3rd interim analysis of the RISA study.
Methods:RISA is an ongoing, single-cohort, non-interventional, multicenter observational study. The individual follow-up period is approximately 24 months. Dosage of EPAG and treatment of patients follows the Summary of Product Characteristic (SmPC) or the routine of treating physicians. Fatigue is assessed at baseline and during the study using the FACIT-Fatigue Scale (Version 4). Annual interim analyses are performed to assess treatment effectiveness and safety. For this interim analysis, an evaluation of patients with prior application of Rituximab is planned.
Results:210 patients received at least one dose of EPAG and completed one post baseline assessment. Mean±SD age was 63.1±17.4 years, median (range) duration of ITP was 5.6 (0.0- 44.9) years, 10% were splenectomized, 52.4% were female, median platelet count (range) at baseline was 33.5x109/L (0.0-270.0), 37.6% reported bleeding complications (any grade) within 12 months prior baseline (WHO °I 30% , °II 4.8% , °III 1.9% , °IV 0% , 1% grade missing), 85.2% received prior ITP therapy, and 81.4% had at least one concomitant disease. At least one pre-treatment was given to 179 patients. More than half received prednisolone (46.2%) or prednisone (9%) and 24.3% dexamethasone or immuno globulins (20%). Rituximab as pre-treatment was given to 2.9% of the patients but further analysis is not possible due to the small number.
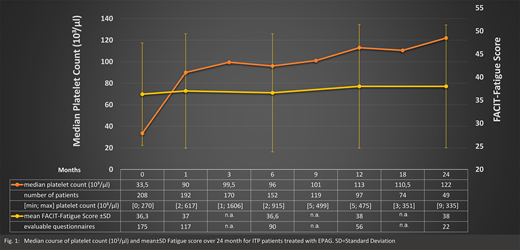
Mean±SD daily dose of EPAG was 45.1±14.4 mg. Treatment with EPAG increased median (range) platelet count to 90x109/L (2.0-617.0) within one month. After two years of treatment median (range) platelet count was 122 (9.0-335.0) (Fig. 1). After one month, 75% of the patients showed treatment response, after 24 months 89 % of the patients exhibited platelet counts above 50x109/L.
At baseline mean±SD FACIT-Fatigue Score was 36.3±11.1 and remained unchanged during the two-year observation period (38.0±13.3) (Fig. 1). In a first subgroup analysis, 55 (31%) of 175 patients with an evaluable questionnaire at baseline suffered from severe fatigue (score <=30) with a mean±SD FACIT-Fatigue Score of 22.4±5.7. Due to the small number of evaluable questionnaires, especially at 12 and 24 months (n=11 and n=4), no reliable results could be provided at this time.
A total of 166 patients (79%) reported any adverse events (AEs) (n=656), 57 patients (27.1%) experienced 126 serious AEs (SAEs) incl. 9 patients (4.3%) with 12 drug related SAEs. A total of 15 patients (7.1%) were reported to have experienced 27 events with fatal outcome. None of the fatal events was assessed causally related to EPAG. Within the first month of treatment 11 of 202 patients (5.4%) reported bleeding complications (any grade) and after two years 2 of 51 (3.9%). Eleven severe thromboembolic events were observed and 2 mild ones (4 severe and one mild related to EPAG).
Discussion:In this 3rd interim analysis it was shown that therapy with EPAG increased the platelet count and reduced bleeding events. No new safety risks were reported despite many concomitant diseases and therapies in these patients. The analysis of the fatigue questionnaire revealed that ITP patients suffer from fatigue, similar to cancer patients and 31% of the patients suffered already from a severe fatigue at baseline. To date, the RISA study could not demonstrate that therapy with EPAG leads to a clinically significant improvement in fatigue. In Germany, ITP patients rarely receive Rituximab prior to EPAG despite their older age and comorbidities. This restrained use of Rituximab is in accordance with the current clinical guidance in Germany. The results in this non-interventional trial are in alignment with the outcomes of other clinical trials with EPAG.
Meyer:Amgen GmbH:Honoraria;Novartis Pharma GmbH:Honoraria;Grifols Germany:Consultancy, Honoraria.Reiser:Celgene:Consultancy, Honoraria;Roche:Consultancy, Honoraria;BMS:Honoraria;CSL Behring:Honoraria.Plath:Novartis Pharma GmbH:Honoraria.Ballerstädt:Novartis Pharma GmbH:Current Employment.Stark-Lorenzen:Novartis Pharma GmbH:Current Employment.Matzdorff:Novartis Oncology:Consultancy, Other: Honoraria paid to institution;Amgen GmbH:Consultancy, Other: Honoraria paid to institution;Grifols Deutschland GmbH:Consultancy, Other: Honoraria paid to institution;Swedish Orphan Biovitrium GmbH:Consultancy, Other: Honoraria paid to institution;UCB Biopharma SRL:Consultancy, Other: Honoraria paid to institution;Roche Pharma AG:Other: Family stockownership.
Rituximab has not been licensed for the treatment of ITP and rituximab is not being, and is not intended to be, used to treat patients in this trial. However, some patients have been pretreated with rituximab outside this trial.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal